Fraxinus excelsior - Ash
GENDER VARIATION IN ASH (FRAXINUS EXCELSIOR L.)
Pierre Binggeli and James Power
ABSTRACT
Although (Fraxinus excelsior L.) is one of the commonest trees found in the British Isles its floral biology is poorly known. This wind-pollinated species is often described as polygamous, trioecious, subdioecious or its sexuality simply recorded as the percentage of trees setting fruits. In May 1989, flowering ash shoots from a natural population were sampled weekly. From both field observations and laboratory measurements the sex expression of single flowers, inflorescences and individuals was determined. Flowers were either male, female or hermaphrodite, whilst inflorescences and individuals usually consisted of a mixture of the three. A few trees were found to be male flowering, but most individuals were hermaphrodite although one sex tended to be dominant. In order to obtain a better understanding of an individual's functional sexuality, an estimate of gender (i.e. the degree of maleness and femaleness of each flowering ash) was obtained. Intra- and inter-tree variation in fruit and seed size was also investigated on some of the fruiting trees.
(This abstract was published in the Proceedings of the Irish Botanists' Meeting, University College Dublin, p. 42)
INTRODUCTION
Although ash (Fraxinus excelsior L.) is one of the common trees of the British Isles, its floral biology is poorly known. This wind-pollinated species is variously described as polygamous, trioecious, subdioecious, or its sexuality is simply recorded as the percentage of trees setting fruits, such statements usually being based on casual observations.
In order to gain a better understanding of the reproductive biology of ash and particularly of its functional sexuality, the morphology and functionality of individual flowers and the sex expression of inflorescences and trees was investigated. Also, a quantitative estimate of gender (i.e. the degree of maleness and femaleness of each flowering ash) was made. Gender differences between juvenile and adult trees, and its impact on fruit and seed weight variation were examined.
FLOWERING IN ASH
Flower Morphology
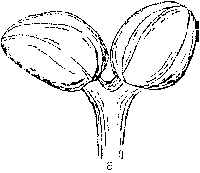
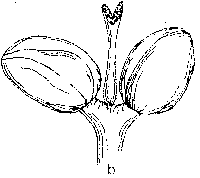
Ash flowers are small and occur in an inflorescence borne on lateral buds. The lack of petals and sepals together with flowering prior to leaf expansion are characteristic features of wind-pollinated species. A typical ash flower is often described as consisting of an ovary and two anthers. However, a large amount of variation can usually be observed and flowers are morphologically either male, female or hermaphrodite (Fig. 1). Morphologically hermaphrodite flowers exhibit considerable variation in the degree of development of the gynoecium or androecium. Anther development can be represented as a continuum ranging from the minute vestigial stamens of some morphologically hermaphrodite flowers, to the plump rounded forms of a male flower.
 |
 |
|
 |
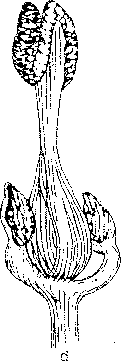 |
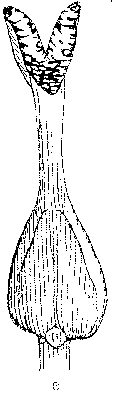 |
Figure 1. Flower types observed on ash (Fraxinus excelsior L.). a) male flower (prior to anthesis), b) hermaphrodite flower with rudimentary gynoecium (functionally male), c) hermaphrodite flower, d) hermaphrodite flower with vestigial anthers (functionally female) and, e) female flower. Flowers and inflorescences at various stages of anthesis are illustrated by Eva Wallander.
Functionality
Unisexual flowers function as male or female; morphologically hermaphrodite flowers may function as either hermaphrodite, male or female. All functional flower types exhibit non-functional equivalents, that is flowers which release no pollen and produce no seeds. Inflorescences and individual trees may be of one type or consist of a mixture of the above.
METHODS
In May 1989, three flowering shoots from 44 trees in a natural population situated on the north coast of Ireland were sampled. The population consisted of a number of adult trees and their putative offspring which had reached sexual maturity. Field observations on the phenology and sex expression of each tree were made on a weekly basis, spanning the flowering period. The number of polleniferous and ovuliferous flowers in the largest inflorescence of each shoot were counted (total number of inflorescences = 130).
The assessment of the functionality of the gynoecium and androecium in morphologically hermaphrodite flowers is problematic. The following procedure was adopted after a preliminary investigation.
For the androecia, a dehisced anther was taken as indicating male functionality. However in most sampled flowers this feature could not be employed. Since all anthers were found to contain some pollen, variation in anther diameter is proposed as a suitable surrogate for functionality.
All trees showing anthers were subjectively assigned to the following three states of anther development:
1. rudimentary anthers
2. intermediate groups
3. fully developed anthers
Two trees were chosen at random from each of the above, and from each of these trees an inflorescence was selected. The diameter of twenty anthers was measured along the longest axis (perpendicular to the filament). A histogram plotted for all trees within this sub-sample produced a bimodal distribution of classes (Fig. 2). This distinct split was useful in defining male functionality; any anther of less than 1.4mm diameter was scored as non-functional.
Figure 2. Size class distribution of anther diameter of ash (stratified random sample of 120 anthers).
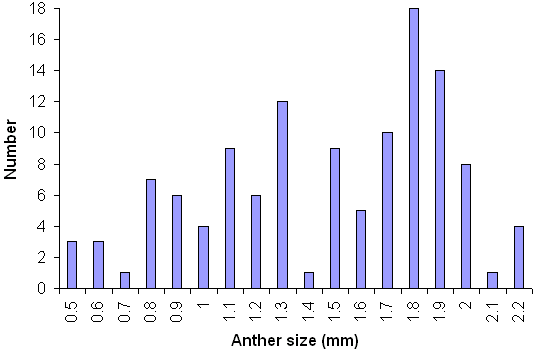
Female functionality was assessed in terms of the degree of development of the pistil; small or misshapen stigmas and styles were assumed to be non functional.
Functional gender can be estimated following the method devised by Lloyd (1980). It is a measure of the potential of a plant contributing genes to the next sexual generation by counts of functional androecial and gynoecial units (note that in ash functionally hermaphrodite flowers will contribute to both female and male function). Gender is then estimated using the following equation:
G = f /[ f +( m * E )]
G denotes functional femaleness varying between 0 and 1, f is the number of functionally female flowers, and m is the number of functionally male flowers. An equivalence factor (E) equates male and female fitness for the population. It is the total number of functionally female flowers in the population divided by the total number of functionally male flowers in the population. In this case a subset of the flower population was used, i.e. the largest inflorescence from each of three shoots per tree.
Fruit and seed dry weight in a random sample of 50 fruits from seven of the trees were measured. These were subsequently analyzed using one way analysis of variance to quantify inter-and intra-tree fruit and seed weight variation.
RESULTS
Functionality of Flowers, Inflorescences and Trees
In the population as a whole morphologically male flowers were the most common type, whilst morphologically female flowers were rare (Table 1). Morphologically hermaphrodite flowers represented 35.5% of the total number of flowers, but most of these functioned as females with only about one quarter viable hermaphrodites. This latter group occurred mainly as the terminal flowers of the inflorescence. The respective frequency of morphologically male, hermaphrodite and female flowers which were non-functional was 0.8%, 1.4% and 0.1%.
Table 1: Type and occurrence of flower morphology and functionality in ash (Fraxinus excelsior L.) (total number of flowers = 14433)
|
Floral morphology |
Flower functionality |
||
|---|---|---|---|
|
Type |
% |
Mode |
% |
|
male |
63.1% |
non-functional |
0.8 |
|
male |
62.3 |
||
|
male |
3.4 |
||
|
hermaphrodite |
35.5% |
male+female |
8.8 |
|
female |
21.9 |
||
|
non-functional |
1.4 |
||
|
female |
1.4% |
female |
1.3 |
|
non-functional |
0.1 |
||
Pure male and pure female flowers, except in 1% of cases, were not seen together on an inflorescence. No radical differences in the sex expression in inflorescences of the same individual were noted.
Sex expression in the trees is first presented in discrete classes (rather than the continuum of the gender estimates) defined simply by the presence or absence of functional flower types. The proportion which were functionally male, female or of mixed sexuality (trees with mixtures of male, female and hermaphrodite flowers) was respectively 20.5%, 11.4% and 68.1%.
Gender Estimate
The distribution of gender among the population is illustrated in Figure 3. Dominantly male individuals (G < 0.1) were identified in 17 trees accounting for 38% of the flowering population in 1989. Dominantly female individuals (G > 0.9) composed 27.3% (12 trees) whilst the 15 individuals of mixed sexuality (0.1 < G < 0.9) comprised 34.1% of the population. There is a continuum in the value of G between the male and female flowering trees within the whole population. When G is expressed for parental and offspring groups separately (Table 2), it is evident that dominantly female trees, whilst hardly represented in the progeny, form the largest subset of the parent trees.
Figure 3. Distribution of gender in an ash population in 1989. Femaleness (G) of each individual is plotted against the rank of the tree within the population for this character.

Comparing the quantitative estimates of gender with field observations of pollen release, seed production and pattern of regeneration revealed good agreement between calculated values of G and these observed gender indicators.
Table 2. Comparison of ash G values between parent and putative progeny.
|
Dominantly male |
Mixed sexuality |
Dominantly female |
|
|---|---|---|---|
|
(G < 0.1) |
(0.1 < G < 0.9) |
(G > 0.9) |
|
|
Population (44 trees) |
38.6% |
34.1% |
27.3% |
|
Parent (21 trees) |
28.6% |
19.0% |
52.4% |
|
Progeny (23 trees) |
47.8% |
47.8% |
4.4% |
Fruit and Seed Weight Variation
Significant differences (p<0.01) were found in the mean dry weight of both fruits and seeds of the seven trees (table 3). A least significant difference test (LSD5%) established that the seeds and fruits of tree A were heavier than the other trees, and that those of tree G were significantly lighter. The mean seed and fruit weights of the remaining five trees were found not to be significantly different. Within and between tree variance in fruit and seed weight accounted for 56% and 44% respectively of the total variance. Corresponding values for seed weight were 62.4% and 37.6%.
Table 3. Functional femaleness (G) and mean fruit and seed dry weight (with standard deviation in parentheses) in seven fruiting ash, based on a sample of 50 fruits.
|
Tree |
G |
Fruit weight(g) |
Seed weight(g) |
|---|---|---|---|
|
A |
1.000 |
0.0795 (0.0137)a |
0.0424 (0.0112)a |
|
B |
0.966 |
0.0689 (0.0113)b |
0.0330 (0.0091)b |
|
C |
0.375 |
0.0623 (0.0089)b |
0.0293 (0.0073)b |
|
D |
0.996 |
0.0610 (0.0123)b |
0.0297 (0.0101)b |
|
E |
1.000 |
0.0594 (0.0079)b |
0.0298 (0.0067)b |
|
F |
1.000 |
0.0575 (0.0169)b |
0.0268 (0.0119)b |
|
G |
0.938 |
0.0453 (0.0082)c |
0.0117 (0.0074)c |
| Different letters within column indicate mean weights are significantly different. | |||
Comparison of G with rank of mean fruit and seed weight reveal that tree A with the heaviest seeds and fruits is pure female (G = 1.000), and that tree G with the lightest seeds and fruits shows an element of male function (G = 0.938). However, tree C, with a G value of 0.375 and hence functionally hermaphrodite, occurs within the group of five trees of statistically equivalent fruit and seed weight. Considering this, and the small size of the sample group, it was concluded that variations in fruit and seed dry weight could not be attributed to gender dominance.
CONCLUSION
Gender in ash is expressed as a continuum between male and female individuals, but is dominated by unisexual trees. Differences in the value of G were most evident between parent and progeny trees. This suggests that with age ash may change their sexual function from predominantly male and hermaphrodite towards femaleness. However, to confirm this trend, it is essential to study gender on a year to year basis.
REFERENCE
Lloyd, D.G. 1980. Sexual strategies in plants. III. A quantitative method for describing the gender of plants. New Zealand Journal of Botany. 18: 103-108.
ACKNOWLEDGMENTS
Hugh Wright's participation in the fieldwork and measurement of seeds and Brian S. Rushton's comments are gratefully acknowledged.